Chinese Journal of Tissue Engineering Research ›› 2019, Vol. 23 ›› Issue (30): 4882-4888.doi: 10.3969/j.issn.2095-4344.1262
Previous Articles Next Articles
Application of recombinant human bone morphogenetic protein-2 in limbs with bone trauma: in-depth investigation of ideal carriers and optimal dosage
- 1Graduate School, 2School of Nursing, Guangxi University of Chinese Medicine, Nanning 530200, Guangxi Zhuang Autonomous Region, China; 3Department of Orthopedics, the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530000, Guangxi Zhuang Autonomous Region, China
-
Received:2019-03-11Online:2019-10-28Published:2019-10-28 -
Contact:Yin Dong, MD, Master’s supervisor, Chief physician, Department of Orthopedics, the People’s Hospital of Guangxi Zhuang Autonomous Region, Nanning 530000, Guangxi Zhuang Autonomous Region, China -
About author:Xie Chengxin, Master candidate, Graduate School, Guangxi University of Chinese Medicine, Nanning 530200,Guangxi Zhuang Autonomous Region, China
CLC Number:
Cite this article
Xie Chengxin, Hu Zhuangming, Wang Wei, Yin Dong, Yu Chengqiang, Wang Chenglong. Application of recombinant human bone morphogenetic protein-2 in limbs with bone trauma: in-depth investigation of ideal carriers and optimal dosage[J]. Chinese Journal of Tissue Engineering Research, 2019, 23(30): 4882-4888.
share this article
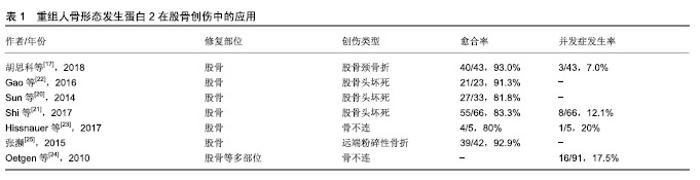
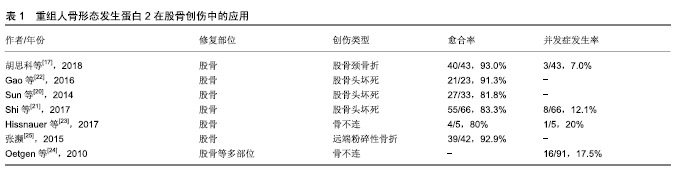
2.1 纳入文献情况 第一作者共检索得到320篇可能相关的文献(数据库:PubMed 92篇、Medline 51篇、Embase 56篇、中国知网59篇、万方35篇及维普27篇),并排除重复文献93篇,相关度低的158篇,可信度低的9篇。最终纳入60篇文献[1-60],包括研究原著43 篇[2,6-8,10-15,17,19-23,26-29,31-40,45-50,53,55-58,60],综述12篇[1,3-5,9,16,18,30,41,44,52,59],病例报告4篇[42-43,51,54],经验交流1篇[25]。纳入文献内容主要包含重组人骨形态发生蛋白2在治疗骨创伤方面的相关研究报道,文章从股骨、胫骨、上肢长骨及手足创伤中的应用及发展趋势及存在的问题方面进行综述。 2.2 重组人骨形态发生蛋白2在四肢骨创伤中的应用 2.2.1 重组人骨形态发生蛋白2在股骨创伤中的应用 股骨颈骨折是一种常见的临床骨折类型,约占全身骨折的3.6%[19],容易发生股骨头缺血性坏死和股骨颈骨不连,治疗需要内固定或人工关节置换。尽管内固定治疗可准确复位,但是患者术后卧床时间较长,相关并发症发生率增加,并且影响骨折愈合,特别是成骨能力缺乏的老年患者容易出现骨不连、畸形愈合或血管坏死,因而治疗效果并不理想。动物实验证实,重组人骨形态发生蛋白2能够促进股骨严重缺损的愈合以及股骨头坏死的成骨及血管生成[13-14]。因此,外科医生尝试利用重组人骨形态发生蛋白2的成骨潜能来促进和加速骨折愈合的过程,详见表1。胡思科等[17]采用闭合复位和空心加压螺钉内固定结合重组人骨形态发生蛋白2治疗股骨颈骨折,结果表明重组人骨形态发生蛋白2促进骨折端愈合,降低股骨头坏死等并发症的发生率。此外,重组人骨形态发生蛋白2还可以提高股骨头坏死骨修复的速度和质量[20],Sun等[20]研究发现添加重组人骨形态发生蛋白2的实验组股骨头存活率高于对照组(81.8% vs. 71.8%),同时提高了保髋手术的优良率(69.7% vs. 64.1%)。Shi等[21]认为适当剂量的重组人骨形态发生蛋白2可作为股骨头坏死手术治疗的辅助治疗。他们还指出重组人骨形态发生蛋白2仍存在风险,会导致一些并 发症,如异位骨化和较高的致癌性[21]。近年来,研究已将其用于治疗儿童及青少年的股骨外伤,Gao等[22]结合骨髓衍生细胞和重组人骨形态发生蛋白2治疗儿童及青少年股骨颈骨折后的骨坏死,术后可减轻髋部疼痛,并能够持续5年以上防止骨坏死进展。一项关于采用重组人骨形态发生蛋白2结合锁定钢板治疗5例儿童及青少年股骨骨不连的研究中显示,有2例患者出现伤口肿胀和过热,1例自发消退,1例感染金黄色葡萄球菌[23]。并且感染金葡菌的患者在应用重组人骨形态发生蛋白2共6个月后骨不连部位仍然没有完整的骨桥形成[23]。另一项研究报道重组人骨形态发生蛋白2在儿童股骨不愈合治疗中会带来一些相关的并发症,如感染和骨筋膜室综合征[24]。由于缺乏更多关于重组人骨形态发生蛋白2在青少年及儿童群体中应用的研究报告,其安全性有待进一步验证。 Baltzer等[15]强调重组人骨形态发生蛋白2可以成功地用于治疗复杂的延迟愈合和可能发生的不愈合,以及作为自体骨移植金标准的等效替代物,并表明重组人骨形态发生蛋白2可在不使用骨导体的情况下诱导和增强骨修复。由此可以看出,重组人骨形态发生蛋白2在治疗股骨颈骨折和预防骨折后血供减少引起的股骨头坏死方面具有一定的优势,已被逐渐应用于股骨头坏死的治疗。还有国内学者将重组人骨形态发生蛋白2用于治疗42例股骨远端粉碎性骨折,患者术后固定牢固,骨折愈合良好[25]。 2.2.2 重组人骨形态发生蛋白2在胫骨创伤中的应用 随着高空作业和运输的快速发展,各种事故引起的高能量性骨折发病率逐年上升,其中胫腓骨干骨折约占全身骨折的9.45%[26]。胫骨骨折由于骨性愈合时间较长,术后康复较慢,部分患者甚至需要行二次翻修手术。据估计,所有骨折患者中骨折不愈合的发生率约为10%,而开放性胫骨骨折患者的不愈合发生率可达到50%[27]。Govender等[28]于2002年发表了首次关于重组人骨形态发生蛋白2治疗胫骨开放性骨折的临床试验,他们将扩髓和非扩髓髓内钉技术分别与质量浓度1.5 g/L重组人骨形态发生蛋白2相结合,发现二者均能加速骨折和伤口愈合,明显降低感染率和二次翻修率,并认为重组人骨形态发生蛋白2具有安全性。随后,重组人骨形态发生蛋白2于2004年被美国食品和药物管理局批准用于治疗开放性胫骨干骨折。然而,也有学者发现重组人骨形态发生蛋白2的作用并不理想。 Aro等[29]对277例患者进行了随机试验,他们将质量浓度1.5 g/L重组人骨形态发生蛋白2联合扩髓内固定组与对照组进行比较,发现前者12周和20周后对愈合无明显促进作用。此外,该研究还发现重组人骨形态发生蛋白2组的深部感染率有增加的趋势,但未见显著性差异(P=0.065)[29],此发现与其他研究认为骨形态发生蛋白可能有助于降低深部感染率的观点有所不同,有学者认为可能与外科手术技术有关[30]。重组人骨形态发生蛋白2联合扩髓型髓内钉治疗胫骨开放性骨折会增加潜在的感染风险,限制了其在非扩髓内钉技术上的应用[31]。Alt等[31]首次专门研究用重组人骨形态发生蛋白2和非扩髓内钉治疗胫骨开放性骨折的患者,发现经重组人骨形态发生蛋白2治疗的患者骨折愈合更快,因此可减少直接二次治疗费用和间接生产力损失,在德国(节省€6 239/人)和英国(节省€4 752/人)使用重组人骨形态发生蛋白2治疗Ⅲ级胫骨开放性骨折是一种更为经济的治疗方法。目前,重组人骨形态发生蛋白2已被欧洲批准用于联合非扩髓内钉治疗开放性胫骨骨折。Lyon等[32]将重组人骨形态发生蛋白2结合可注射磷酸钙基质载体治疗闭合性胫骨干骨折,研究发现骨折愈合没有显著改善。然而,治疗组相比对照组发生严重并发症、水肿和深静脉血栓形成的数量却显著增加[32]。该项研究认为重组人骨形态发生蛋白2结合物缺乏疗效,但没有否定重组人骨形态发生蛋白2的骨诱导作用[32]。最近,有研究报道植入重组人骨形态发生蛋白2治疗先天性胫骨假关节骨折患者有助于缩短愈合时间,但并不能确保愈合发生[33]。Stiel等[34]将重组人骨形态发生蛋白2应用于治疗先天性胫骨假关节、胫骨骨不连等疾病,他们认为血肿、骨筋膜室综合征、深部感染是与使用重组人骨形态发生蛋白2相关的惟一并发症。 Barcak等[30]总结了重组人骨形态发生蛋白2在胫骨开放性骨折中的应用:①重组人骨形态发生蛋白2可能有助于开放性胫骨骨折的愈合;②重组人骨形态发生蛋白2在重度开放性创伤中比在轻度开放性创伤中更有效;③有矛盾的证据表明重组人骨形态发生蛋白2提高了感染率。此外,笔者也发现许多学者对于使用重组人骨形态发生蛋白2后相关并发症方面存在一定的争议[28,32,34]。尽管重组人骨形态发生蛋白2在治疗胫骨创伤疾病方面表现出良好的骨诱导性,但对于治疗效果仍存在不确定性,需要更多研究为其广泛应用提供充分的临床依据。 2.2.3 重组人骨形态发生蛋白2在上肢长骨创伤中的应用 由于缺少重组人骨形态发生蛋白2治疗肱骨创伤的具体研究数据,对其疗效的评估还存在局限性,目前仅有少数患者在治疗过程中添加重组人骨形态发生蛋白2作为辅助治疗。Konda等[35]在一项关于治疗肱骨骨不连的研究中,对其中9例患者实施骨形态发生蛋白2辅助治疗,研究结果显示无统计学差异(P=0.42)。谢纪宝等[36]使用β-磷酸三钙人工骨联合重组人骨形态发生蛋白2填充治疗肱骨近端良性肿瘤刮除后的骨缺损,在所有病例中均未发现排异反应、伤口感染、骨折不愈合等情况。Von Ruden等[37]不建议将重组人骨形态发生蛋白2作为治疗无菌性桡骨、尺骨骨不连的辅助手段,因重组人骨形态发生蛋白2与自体骨移植疗效相当,且成本较高。 2.2.4 重组人骨形态发生蛋白2在手足创伤中的应用 重组人骨形态发生蛋白2的应用显著降低了持久性骨折不愈合的整体治疗成本,为患者减少住院时间并较早恢复劳动能力,其在手足创伤中的推广具有非常可观的前景。陈启刚等[38]将15例跟骨骨折患者经锁定板结合重组人骨形态发生蛋白2治疗,术后骨折均完全愈合,无畸形愈合。Rearick等[39]首次报道了重组人骨形态发生蛋白2在前、中足手术以及足踝骨折和截骨不连中的应用,结果显示在平均愈合时间111 d(15.9周)内愈合率达到95%。但在一项治疗手腕和手部骨不连的研究中,重组人骨形态发生蛋白2没有产生较高的愈合率[40]。虽然有学者认为应用重组人骨形态发生蛋白2能够降低并发症发生率[39-40],但也有研究报道在手足及四肢创伤治疗中使用骨形态发生蛋白会导致异位骨形成[41],甚至会导致持续性炎症反应及骨吸收[42]。 2.3 发展趋势及问题 2.3.1 发展趋势 随着细胞生物学和骨组织工程学的发展,越来越多的研究投入到骨再生技术中。骨形态发生蛋白对于维持骨量和肌腱、韧带或软骨的发育是必不可少的[43]。目前,骨形态发生蛋白在关节炎治疗、骨折治疗、骨不连及骨缺损治疗、股骨头坏死治疗及基因治疗等方面积累了许多临床经验[44]。重组人骨形态发生蛋白2,7均已通过美国食品和药物管理局临床批准许可用于商业治疗,并且有文献报道重组人骨形态发生蛋白2的临床疗效比重组人骨形态发生蛋白7更为理想[45-46]。重组人骨形态发生蛋白2的应用代表了避免大范围骨移植手术的未来,为了更好地控制这种功能强大的蛋白质的作用和释放,应该对理想的载体进行进一步的研究。研究表明,含有胶原结合域的重组人骨形态发生蛋白2与含有胶原的骨修复材料相结合,具有良好的稳定性,缓慢释放,并且在较长时间内具有较强的骨诱导活性[8]。不仅可以显著提高骨损伤的修复能力,同时也显著减少重组人骨形态发生蛋白2用量,降低了使用风险[8]。由此可见,重组人骨形态发生蛋白2的有效性和安全性表现为载体依赖性,选择合适的载体尤为关键。已有多项研究在重组人骨形态发生蛋白2联合载体方面积累了一定临床经验[29,32,36,47-49],丰富了重组人骨形态发生蛋白2载体的可选择性。研究发现工程化大肠杆菌能提供另一种生产重组蛋白的系统,并证明大肠杆菌衍生的骨形态发生蛋白2生产成本相对较低[50]。 2.3.2 并发症 使用重组人骨形态发生蛋白2后并发症的发生率可高达39%[34],例如全身毒性、过度骨生长、创面引流增加、过敏反应及致癌性[4,21,31-32,34,51]。因此,使用重组人骨形态发生蛋白2必须将其潜在益处与潜在问题相互权衡。几组文献中报道重组人骨形态发生蛋白2会导致伤口愈合延迟、增加引流量、血肿疼痛、神经炎、神经功能缺损、感染及异位骨化造成的神经压迫[29,39,52-53]。Matthews等[54]首次报道了创伤部位发生周围神经功能障碍与使用重组人骨形态发生蛋白2直接相关,而非水肿或肿块压迫所致。相反,有学者认为重组人骨形态发生蛋白2的使用不会增加感染率和二次手术率[55]。一项涉及4 000多例患者的研究提示,骨形态发生蛋白会增加良性肿瘤的风险,但不会增加恶性肿瘤的风险[56]。目前,仍缺少重组人骨形态发生蛋白2在长骨中使用后癌症风险增加的可靠数据[37]。 2.3.3 成本及剂量 重组人骨形态发生蛋白2的临床应用还存在高成本的缺点。Ronga等[41]建议降低骨形态发生蛋白生产成本,并在选定的疑难病例中使用,可带来更好的成本效益。笔者认为还可以通过降低重组人骨形态发生蛋白2的使用剂量来减少患者治疗费用。浓度是影响骨形态发生蛋白效应的关键调控因素之一,目前推荐的骨形态发生蛋白2,7使用剂量是天然浓度的1 000倍以上[30]。重组人骨形态发生蛋白2在植入早期大量释放并迅速降解和吸收,低浓度重组人骨形态发生蛋白2难以维持有效浓度持续刺激靶细胞发挥其骨诱导性,而高浓度(超过40 mg)的重组人骨形态发生蛋白2则会增加癌症等并发症的发生[57]。目前界内学者对于最佳剂量的选择还未达成一致意见,Richards等[33]认为重组人骨形态发生蛋白2剂量为4.2-8.4 mg最合适。有文献报道,骨髓浓缩物能协同增强重组人骨形态发生蛋白2的骨诱导功能,可使重组人骨形态发生蛋白2的用量减少一半,从而减少不良反 应,有效地诱导骨形成[58]。 "
| [1]Pilipchuk SP, Plonka AB, Monje A, et al. Tissue engineering for bone regeneration and osseointegration in the oral cavity. Dent Mater. 2015;31(4):317-338.[2]常晓朋,陈涛,赵寅,等.骨形态发生蛋白2和转化生长因子β2协同促进骨髓间充质干细胞成骨分化[J].中国组织工程研究,2019, 23(1):1-6.[3]Trento GS, Carvalho PHA, Macedo DV, et al. Titanium mesh associated with rhBMP-2 in alveolar ridge reconstruction. Int J Oral Maxillofac Surg. 2018. DOI:10.1016/j.ijom.2018.09.015[4]Poon B, Kha T, Tran S, et al. Bone morphogenetic protein-2 and bone therapy: successes and pitfalls. J Pharm Pharmacol. 2016;68(2):139-147.[5]Mussano F, Ciccone G, Ceccarelli M, et al. Bone morphogenetic proteins and bone defects: a systematic review. Spine (Phila Pa 1976). 2007;32(7):824-830.[6]Hreha J, Krell E S, Bibbo C. Role of Recombinant human bone morphogenetic protein-2 on hindfoot arthrodesis. Foot Ankle Clin. 2016;21(4):793-802.[7]Katayama Y, Matsuyama Y, Yoshihara H, et al. Clinical and radiographic outcomes of posterolateral lumbar spine fusion in humans using recombinant human bone morphogenetic protein-2: an average five-year follow-up study. Int Orthop. 2009;33(4):1061-1067.[8]刘启省,张东刚.重组人骨形态发生蛋白2/骨修复材料的制备与性能[J].中国组织工程研究,2016,20(38):5664-5671.[9]Herford AS. The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in maxillofacial trauma. Chin J Traumatol. 2017;20(1):1-3.[10]Kwak EJ, Cha IH, Nam W, et al. Effects of locally administered rhBMP-2 and bisphosphonate on bone regeneration in the rat fibula. Oral Dis. 2018;24(6): 1042-1056.[11]Carlisle P, Guda T, Silliman DT, et al. Localized low-dose rhBMP-2 is effective at promoting bone regeneration in mandibular segmental defects. J Biomed Mater Res B Appl Biomater. 2018. DOI: 10.1002/jbm.b.34241.[12]Nam JW, Kim HJ. Stepwise verification of bone regeneration using recombinant human bone morphogenetic protein-2 in rat fibula model. J Korean Assoc Oral Maxillofac Surg. 2017; 43(6):373-387.[13]Pan ZX, Zhang HX, Wang YX, et al. Effect of recombinant human bone morphogenetic protein 2/poly-lactide-co-glycolic acid (rhBMP-2/PLGA) with core decompression on repair of rabbit femoral head necrosis. Asian Pac J Trop Med. 2014; 7(11):895-899.[14]Skelly JD, Lange J, Filion TM, et al. Vancomycin-bearing synthetic bone graft delivers rhBMP-2 and promotes healing of critical rat femoral segmental defects. Clin Orthop Relat Res. 2014;472(12):4015-4023.[15]Baltzer AW, Ostapczuk MS, Stosch D, et al. The use of recombinant human bone morphogenetic protein-2 for the treatment of a delayed union following femoral neck open-wedge osteotomy. Orthop Rev (Pavia). 2012;4(1):e4.[16]Lykissas M, Gkiatas I. Use of recombinant human bone morphogenetic protein-2 in spine surgery. World J Orthop. 2017;8(7):531-535.[17]胡思科,罗任,劳世高,等.经空心加压螺钉隧道重组人骨形态发生蛋白2植入术治疗股骨颈骨折的临床观察[J].现代医院, 2018, 18(5):747-749,752.[18]刘军,王刚,谷贵山,等.开放性骨折的治疗[J].中国矫形外科杂志, 2007,15(24):1878-1881.[19]苏晨晨,张文生,刘世平,等.中青年股骨颈骨折空心螺钉内固定术后股骨头坏死的相关因素分析[J].局解手术学杂志,2018,27(9): 665-669.[20]Sun W, Li Z, Gao F, et al. Recombinant human bone morphogenetic protein-2 in debridement and impacted bone graft for the treatment of femoral head osteonecrosis. PLoS One. 2014;9(6):e100424.[21]Shi L, Sun W, Gao F, et al. Heterotopic ossification related to the use of recombinant human BMP-2 in osteonecrosis of femoral head. Medicine (Baltimore). 2017;96(27):e7413.[22]Gao F, Sun W, Guo W, et al. Combined with bone marrow-derived cells and rhbmp-2 for osteonecrosis after femoral neck fractures in children and adolescents: a case series. Sci Rep. 2016;6:30730.[23]Hissnauer TN, Stiel N, Babin K, et al. Recombinant human bone morphogenetic protein-2 (rhbmp-2) for the treatment of nonunion of the femur in children and adolescents: a retrospective analysis. Biomed Res Int. 2017;2017:3046842.[24]Oetgen ME, Richards BS. Complications associated with the use of bone morphogenetic protein in pediatric patients. J Pediatr Orthop. 2010;30(2):192-198.[25]张濒.应用解剖锁定钢板及rhBMP-2治疗股骨远端粉碎性骨折的疗效观察[C].中国转化医学和整合医学学术交流会(上海站), 2015. [26]李飞,庞彬.开放性胫骨骨折内固定术后感染的危险因素和病原菌分析[J].解放军医药杂志,2018,30(11):55-57, 61.[27]Bosse MJ, Mackenzie EJ, Kellam JF, et al. An analysis of outcomes of reconstruction or amputation after leg-threatening injuries. N Engl J Med. 2002;347(24): 1924-1931.[28]Govender S, Csimma C, Genant HK, et al. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002; 84-A(12):2123-2134.[29]Aro H T, Govender S, Patel AD, et al. Recombinant human bone morphogenetic protein-2: a randomized trial in open tibial fractures treated with reamed nail fixation. J Bone Joint Surg Am. 2011;93(9):801-808.[30]Barcak EA, Beebe MJ. Bone morphogenetic protein: is there still a role in orthopedic trauma in 2017? Orthop Clin North Am. 2017;48(3):301-309.[31]Alt V, Borgman B, Eicher A, et al. Effects of recombinant human Bone Morphogenetic Protein-2 (rhBMP-2) in grade III open tibia fractures treated with unreamed nails-A clinical and health-economic analysis. Injury. 2015;46(11):2267-2272.[32]Lyon T, Scheele W, Bhandari M, et al. Efficacy and safety of recombinant human bone morphogenetic protein-2/calcium phosphate matrix for closed tibial diaphyseal fracture: a double-blind, randomized, controlled phase-II/III trial. J Bone Joint Surg Am. 2013;95(23):2088-2096.[33]Richards BS, Anderson TD. rhBMP-2 and intramedullary fixation in congenital pseudarthrosis of the tibia. J Pediatr Orthop. 2018;38(4):230-238.[34]Stiel N, Hissnauer TN, Rupprecht M, et al.Evaluation of complications associated with off-label use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in pediatric orthopaedics. J Mater Sci Mater Med. 2016;27(12):184.[35]Konda SR, Davidovitch RI, Egol KA. Initial surgical treatment of humeral shaft fracture predicts difficulty healing when humeral shaft nonunion occurs. HSS J. 2016;12(1):13-17.[36]谢纪宝,郑永茂,李云飞,等.β-磷酸三钙人工骨联合rhBMP-2重建肱骨近端良性骨肿瘤及骨瘤样病变刮除[J].华夏医学,2018, 31(1):39-41.[37]Von Ruden C, Morgenstern M, Hierholzer C, et al. The missing effect of human recombinant bone morphogenetic proteins BMP-2 and BMP-7 in surgical treatment of aseptic forearm nonunion. Injury. 2016;47(4):919-924.[38]陈启刚,胡永军,胡海,等.锁定接骨板结合重组人骨形态发生蛋白骨修复材料治疗跟骨骨折的临床分析[J].安徽医药,2018,22(4): 703-706.[39]Rearick T, Charlton TP, Thordarson D. Effectiveness and complications associated with recombinant human bone morphogenetic protein-2 augmentation of foot and ankle fusions and fracture nonunions. Foot Ankle Int. 2014;35(8): 783-788.[40]Rice I, Lubahn J D.Use of bone morphogenetic protein-2 (rh-BMP-2) in treatment of wrist and hand nonunion with comparison to historical control groups. J Surg Orthop Adv. 2013;22(4):256-262.[41]Ronga M, Fagetti a, Canton G, et al. Clinical applications of growth factors in bone injuries: experience with BMPs. Injury. 2013;44 Suppl 1:S34-S39.[42]Ritting AW, Weber EW, Lee MC. Exaggerated inflammatory response and bony resorption from BMP-2 use in a pediatric forearm nonunion. J Hand Surg Am. 2012;37(2):316-321.[43]Patel AD. Bone morphogenetic proteins in orthopaedic trauma: recent clinical findings with human bone morphogenetic protein-2 (rhBMP-2). Curr Med Res Opin. 2006;22 Suppl 1:S1-S5.[44]汤显能,陈跃平,章晓云.骨与软骨组织工程中骨形态发生蛋白的研究与临床应用[J].中国组织工程研究,2019,23(4):591-596.[45]Das SP, Ganesh S, Pradhan S, et al. Effectiveness of recombinant human bone morphogenetic protein-7 in the management of congenital pseudoarthrosis of the tibia: a randomised controlled trial. Int Orthop. 2014;38(9): 1987-1992.[46]Papanagiotou M, Dailiana ZH, Karachalios T, et al. Heterotopic ossification after the use of recombinant human bone morphogenetic protein-7. World J Orthop. 2017;8(1): 36-41.[47]Li R, Ma Y, Zhang Y, et al. Potential of rhBMP-2 and dexamethasone-loaded Zein/PLLA scaffolds for enhanced in vitro osteogenesis of mesenchymal stem cells. Colloids Surf B Biointerfaces. 2018;169:384-394.[48]Bae EB, Park KH, Shim JH, et al. Efficacy of rhBMP-2 loaded PCL/beta-TCP/bdECM scaffold fabricated by 3D printing technology on bone regeneration. Biomed Res Int. 2018; 2018:2876135.[49]Zhou P, Xia Y, Cheng X, et al. Enhanced bone tissue regeneration by antibacterial and osteoinductive silica-HACC-zein composite scaffolds loaded with rhBMP-2. Biomaterials. 2014;35(38):10033-10045.[50]Kuroiwa Y, Niikura T, Lee SY, et al. Escherichia coli-derived BMP-2-absorbed beta-TCP granules induce bone regeneration in rabbit critical-sized femoral segmental defects. Int Orthop. 2018. DOI: 10.1007/s00264-018-4079-4.[51]Boraiah S, Paul O, Hawkes D, et al. Complications of recombinant human BMP-2 for treating complex tibial plateau fractures: a preliminary report. Clin Orthop Relat Res. 2009; 467(12):3257-3262.[52]Carragee EJ, Hurwitz EL, Weiner BK. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: emerging safety concerns and lessons learned. Spine J. 2011;11(6):471-491.[53]Smucker JD, Rhee JM, Singh K, et al. Increased swelling complications associated with off-label usage of rhBMP-2 in the anterior cervical spine. Spine (Phila Pa 1976). 2006; 31(24):2813-2819.[54]Matthews JR, Margolis DS, Wu E, et al. Brachial plexopathy following use of recombinant human bmp-2 for treatment of atrophic delayed union of the clavicle. JBJS Case Connect. 2015;5(3):e81-e85.[55]Chan D, Garland J, Infante A, et al.Wound complications associated with bone morphogenetic protein-2 in orthopaedic trauma surgery. J Orthop Trauma. 2014;28(10):599-604.[56]Lad SP, Bagley JH, Karikari IO, et al. Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery. 2013;73(3):440-449.[57]Carragee EJ, Chu G, Rohatgi R, et al. Cancer risk after use of recombinant bone morphogenetic protein-2 for spinal arthrodesis. J Bone Joint Surg Am. 2013;95(17):1537-1545.[58]Egashira K, Sumita Y, Zhong W, et al. Bone marrow concentrate promotes bone regeneration with a suboptimal-dose of rhBMP-2. PLoS One. 2018;13(1): e0191099.[59]De Biase P, Capanna R. Clinical applications of BMPs. Injury. 2005;36 Suppl 3:S43-S46.[60]Takemoto R, Forman J, Taormina DP, et al. No advantage to rhBMP-2 in addition to autogenous graft for fracture nonunion. Orthopedics. 2014;37(6):e525-530. |
| [1] | Liu Lihua, Sun Wei, Wang Yunting, Gao Fuqiang, Cheng Liming, Li Zirong, Wang Jiangning. Type L1 steroid-induced osteonecrosis of the femoral head through femoral head and neck junction decompression by fenestration: a single-center prospective clinical study [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 906-911. |
| [2] | Yuan Xinping, Shao Yanbo, Wu Chao, Wang Jianling, Tong Liangcheng, Li Ying. Accuracy of target bone segments in personalized differential modeling and simulation of CT scanning parameters at fracture end [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 912-916. |
| [3] | Liu Zhao, Xu Xilin, Shen Yiwei, Zhang Xiaofeng, Lü Hang, Zhao Jun, Wang Zhengchun, Liu Xuzhuo, Wang Haitao. Guiding role and prospect of staging and classification combined collapse prediction method for osteonecrosis of femoral head [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 929-934. |
| [4] | Li Shibin, Lai Yu, Zhou Yi, Liao Jianzhao, Zhang Xiaoyun, Zhang Xuan. Pathogenesis of hormonal osteonecrosis of the femoral head and the target effect of related signaling pathways [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(6): 935-941. |
| [5] | Zheng Xiaolong, He Xiaoming, Gong Shuidi, Pang Fengxiang, Yang Fan, He Wei, Liu Shaojun, Wei Qiushi. Bone turnover characteristics in patients with alcohol-induced osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(5): 657-661. |
| [6] | Ma Ziyue, Ju Xiaochen, Zhang Lei, Sun Rongxin. Tendon-bone healing in anterior cruciate ligament reconstruction with and without remnant preservation [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(4): 582-587. |
| [7] | Zeng Xianghong, Liang Bowei. A new strategy for the treatment of osteonecrosis of the femoral head [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(3): 431-437. |
| [8] | Lin Wang, Wang Yingying, Guo Weizhong, Yuan Cuihua, Xu Shenggui, Zhang Shenshen, Lin Chengshou. Adopting expanded lateral approach to enhance the mechanical stability and knee function for treating posterolateral column fracture of tibial plateau [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(24): 3826-3827. |
| [9] | Zhang Shengmin, Cao Changhong, Liu Chao. Adipose-derived stem cells integrated with concentrated growth factors prevent bisphosphonate-related osteonecrosis of the jaws in SD rats [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(19): 2982-2987. |
| [10] | Zhou Yi, Chen Yueping, Zhang Xiaoyun, Lai Yu, Liao Jianzhao, Li Shibin. An exploration on mechanism of Shengyu Decoction in treating osteonecrosis of the femoral head based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2687-2696. |
| [11] | Xin Pengfei, Ke Mengnan, Zhang Haitao, Urishana, Li Ziqi, Zhuang Zhikun, Wei Qiushi, He Wei. Common mechanism of Chinese herbs for promoting blood circulation and removing blood stasis in the treatment of osteonecrosis of the femoral head: an analysis based on network pharmacology [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(17): 2727-2733. |
| [12] | Meng Maohua, Li Ying, Chen Xin, Cheng Lu, Dong Qiang. Effects and mechanisms of enamel matrix derivatives on osteogenic differentiation of bone marrow mesenchymal stem cells [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(13): 2108-2113. |
| [13] | Xia Sijie, Liao Qi. Low-intensity pulsed ultrasound in treatment of fractures: a systematic review and meta-analysis [J]. Chinese Journal of Tissue Engineering Research, 2021, 25(12): 1944-1950. |
| [14] |
Wang Tiantian, Wang Jianzhong.
Application and prospect of bone marrow mesenchymal stem cells in the
treatment of early femoral head necrosis |
| [15] | Han Xinguang, Yin Zhijiang, Wang Xiangcheng, Yang Chenyu, Shang Jian. Effectiveness and safety of modified sliding chute bone graft plus hollow screw fixation for tibiotalar joint fusion [J]. Chinese Journal of Tissue Engineering Research, 2020, 24(6): 839-842. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||